Prostate cancer begins when cells in the prostate gland start to grow out of control. These cancerous cells may remain in the prostate or metastasize and spread to other parts of the body such as the bones, lymph nodes, liver, or lungs.
Prostate-specific membrane antigen (PSMA) is a protein found on the surface of prostate cancer cells. PSMA-targeting can be used to locate, identify, or treat cancerous cells in both the prostate as well as cancerous cells that have metastasized in other organs. This targeting can involve attaching a radionuclide, a particle that gives off radiation, to different molecular agents, most often monoclonal antibodies or small molecule targeting agents (also known as peptides, ligands, or inhibitors). This combination attaches to the PSMA receptors located in the cancer cells.
PSMA positron emission tomography (PET) scans are an imaging technique approved by the U.S. Food and Drug Administration (FDA). PSMA PET is able to precisely locate prostate cancer cells using a radioactive imaging agent that binds to prostate cancer cells to help localize them. This drug is injected into the body and attaches itself to PSMA proteins expressed by prostate cancer patients. The PET scan is then able to detect and pinpoint the prostate cancer tumors.
Weill Cornell Medicine was one of the first centers in the United States offering this technology for patients. PSMA PET is able to identify whether the cancer has spread beyond the prostate gland with higher accuracy than other imaging methods.
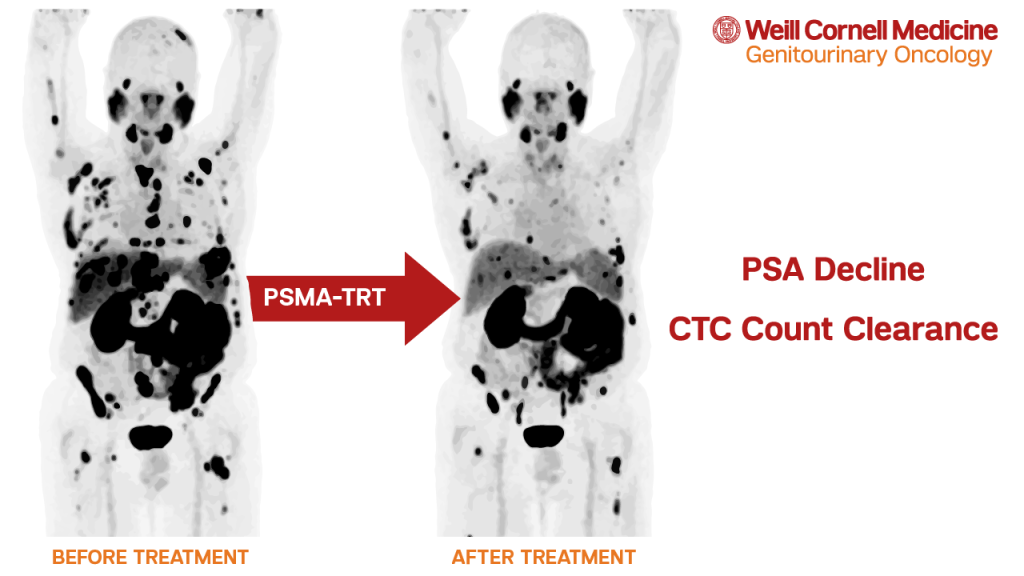
The team at the Weill Cornell Medicine Genitourinary (GU) Oncology Program has been a pioneer for PSMA-targeted therapies for many years. PSMA-targeted radionuclide therapies lead the combination of radionuclide and molecular agents directly to the PSMA cell receptors. The ability for the targeting agents and the PSMA receptors to join together provides this therapy the ability to precisely target prostate cancer cells.
In March 2022, the FDA approved 177Lu-PSMA-617 (also known as Lu 177 vipivotide tetraxetan or Pluvicto) for the treatment of patients with metastatic castration-resistant prostate cancer. Dr. Scott Tagawa, director of the Weill Cornell Medicine Genitourinary (GU) Oncology Program, was a member of the steering committee for the trial evaluating this treatment that led to the approval.
“The first availability of tumor-targeted radionuclide therapy on a commercial basis will allow patients with more limited resources that might not have been able to travel for a clinical trial or overseas to receive the benefit of this treatment. The first successful phase 3 trial allows us in research to optimize the treatment, study it in earlier disease states, and explore combinations with other therapies with scientific merit. Even after this approval, I encourage clinical trial participation and/or referrals.”
Weill Cornell Medicine will be one of the first centers able to offer this therapy to patients immediately for both our existing patients and those who may be referred to us if their local provider doesn’t immediately have access to this therapy.
Our team continues to lead and participate in a number of clinical trials aimed at ongoing testing and research for additional PSMA-targeted imaging and treatment. We are one of few centers in the world currently able to provide treatment plans that involve both PSMA-PET imaging and multiple PSMA-targeted therapies for our patients, as well as the opportunity to participate in these types of clinical trials in order to further develop PSMA technology.
The Weill Cornell Medicine Genitourinary (GU) Oncology Program provides top-notch care, knowledge, and expertise for our patients. We offer new patient appointments, second opinions, and ongoing care for people with genitourinary cancers, including prostate cancer. To learn more or to make an appointment with one of our physicians, please call us at 646-962-2072. If interested in a clinical trial, please email us at guonc@med.cornell.edu.