The 2023 American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU) brought together members of the cancer care and research community to share new, innovative findings in the study, diagnosis, and treatment of GU malignancies. This year, ASCO GU was held in a hybrid format, taking place live in San Francisco from February 16-18, as well as virtually online.
A number of members of the Weill Cornell Medicine (WCM) Genitourinary (GU) Oncology Program presented or were involved with new research findings shared at the conference, as well as trial-in-progress updates for some of the clinical trials currently underway and open to accrual at Weill Cornell Medicine and NewYork-Presbyterian Hospital (NYP).
Our team’s contributions included both scientific and treatment advancements that are leading the way for more targeted therapies and improved outcomes for patients, as well as current trial-in-progress updates on ongoing studies.
Some of our the highlights include Dr. Scott Tagawa presenting on a combination treatment of PSMA-targeted therapy and hormonal therapy for certain patients with prostate cancer, Dr. Jones Nauseef presenting details on two different Weill Cornell Medicine studies involving an investigational therapy for metastatic castration-resistant prostate cancer and a collaboration genomic alterations project, and Dr. Cora Sternberg’s involvement with two clinical trials for prostate cancer and bladder cancer which had follow up results presented.
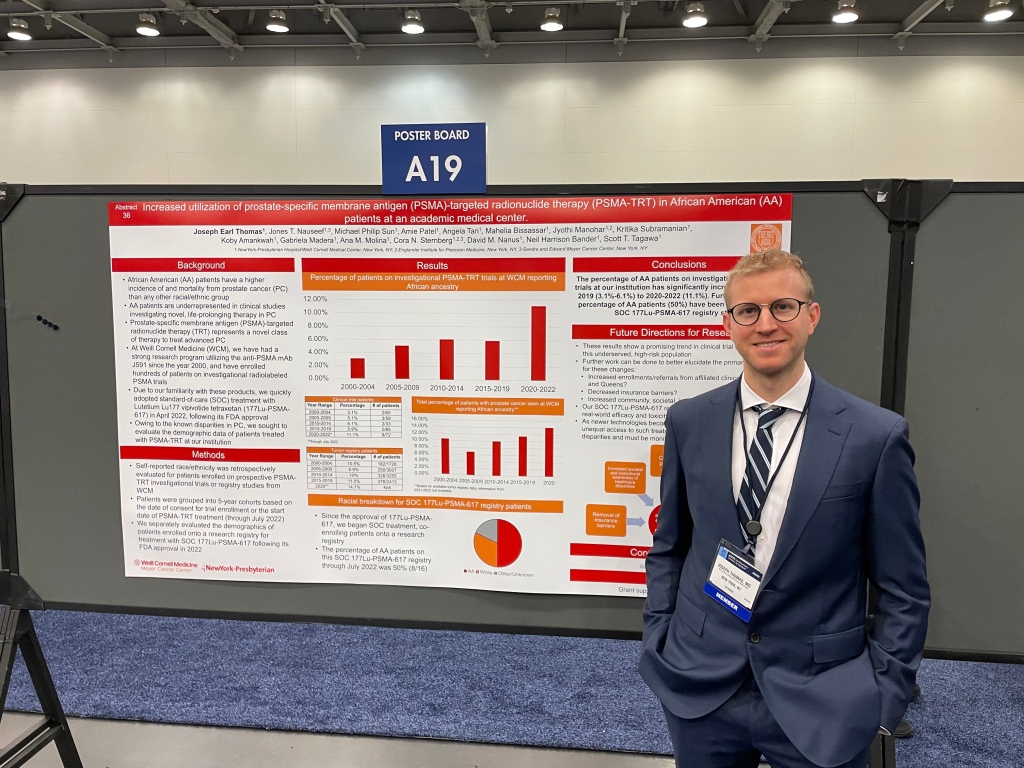
Two of our Hematology & Oncology Fellows also presented research updates from Weill Cornell Medicine studies. Dr. Joseph Thomas shared insights from demographic patient data for those treated on prostate-specific membrane antigen (PSMA)-targeted radionuclide clinical trials and Dr. Michael Sun presented on an investigational treatment combination for metastatic prostate cancer.
Additionally, Dr. David Nanus served on an expert panel for a session on clinical decision-making for prostate cancer, “Novel Guidelines for PSMA PET Staging of Disease: When to Follow and When to Not.”
Read more specifics below on our various team members’ involvement and research from the conference.
Prostate Cancer
Dr. Scott Tagawa presented exciting new research from Weill Cornell Medicine/NewYork-Presbyterian Hospital showing that a unique combination of PSMA-targeted therapy and hormonal therapy delayed metastases in a subset of patients with prostate cancer.

A phase I/II clinical trial evaluating the investigational therapy 225Ac-J591 is underway at Weill Cornell Medicine for metastatic castration-resistant prostate cancer. Dr. Jones Nauseef explains the study aims which were presented as part of a trial-in-progress update: https://bit.ly/3XwUE02. This trial, which enrolls patients whether or not they have previously received 177Lu-PSMA, is ongoing.

Results from a phase 3 clinical trial showed improved overall survival in metastatic hormone-sensitive prostate cancer patients receiving darolutamide, androgen-deprivation therapy (ADT) and chemotherapy. Dr. Cora Sternberg shared takeaways from this research which led to the United States Food and Drug Administration (FDA) approval of this combination in August 2022: https://bit.ly/3xkfQeO

Dr. Timothy McClure explains a focal therapy prostate cancer treatment that is being evaluated in a clinical trial at Weill Cornell Medicine in combination with lower-dose radiotherapy: https://bit.ly/3E38YXi

Weill Cornell Medicine Hematology & Oncology Fellow Dr. Joseph Thomas shared details from a research registry analyzing demographic patient data for those treated on prostate-specific membrane antigen (PSMA)-targeted radionuclide clinical trials: https://bit.ly/3xsFVbD

Dr. Himanshu Nagar shared information about an ongoing phase 2 clinical trial comparing 4 weeks to 2 weeks of MRI-guided radiotherapy after prostate cancer surgery: https://bit.ly/3HZk14W. The SHORTER trial is currently open to enrollment at Weill Cornell Medicine/NewYork-Presbyterian Hospital.

Weill Cornell Medicine Hematology & Oncology Fellow Dr. Michael Sun shared preliminary results from Weill Cornell Medicine research evaluating a treatment combination of immunotherapy, anti-androgen medication, and PSMA-targeted radionuclide therapy for men with metastatic prostate cancer: https://bit.ly/3YKBxk4. This trial has entered a randomized phase and is enrolling patients.

Dr. Ariel Marciscano breaks down Weill Cornell Medicine research studying irradiated prostate cancer tumors in an effort to analyze and understand the changes in the tumor and microenvironment after radiation treatment: https://bit.ly/40YDVWu https://bit.ly/3I0CBtj

Members of the Weill Cornell Medicine Englander Institute for Precision Medicine collaborated with the New York Genome Institute and genomic company Isabl to identify both known and unexpected genomic alterations in prostate cancer patients. Dr. Jones Nauseef shares insights: https://bit.ly/3xk21NC

A Weill Cornell clinical trial led by Dr. Himanshu Nagar was presented comparing two stereotactic body radiation therapy (SBRT) sessions to five SBRT sessions for prostate cancer: https://bit.ly/3E6erMS.

Weill Cornell Medicine Urology resident Dr. Alec Zhu discusses new Weill Cornell Medicine research he presented comparing cryoablation treatment outcomes between men with MRI-visible and MRI-invisible prostate cancer. https://bit.ly/3YQPcpG

Bladder Cancer
Dr. Cora Sternberg breaks down long-term follow-up results on a clinical trial evaluating avelumab as maintenance therapy for bladder cancer: https://bit.ly/3XvsyC8

Dr. Himanshu Nagar shares more about a clinical trial comparing immunotherapy alone to immunotherapy plus radiation therapy for bladder cancer. https://bit.ly/3E38YXi. This trial is open across the country, with patients receiving pembrolizumab with or without radiation.

Additionally, updates to three cohorts of the TROPHY-U-01 study evaluating the antibody-drug conjugate sacituzumab govitecan were presented by a team of authors including Drs. Tagawa and Sternberg at Weill Cornell Medicine. Cohort 1 of the study enrolled patients with progressive advanced urothelial carcinoma (UC) despite platinum chemotherapy and immune checkpoint inhibitors. Initial results led to an accelerated approval by the U.S. Food and Drug Administration (FDA). In an update presented at GU ASCO 2023, the research demonstrated that efficacy was maintained and no new toxicity signals emerged despite longer follow up. In Cohort 2, patients with advanced UC who were not candidates for cisplatin or carboplatin chemotherapy following immune checkpoint inhibitors received sacituzumab govitecan which demonstrated a 32% objective response rate. Cohort 3 enrolled patients with advanced UC following platinum chemotherapy, treating them with sacituzumab govitecan and pembrolizumab, leading to a 41% overall response rate. Ongoing enrollment is testing this drug in earlier lines of therapy with different combinations. Dr. Scott Tagawa spoke about the TROPHY-U-01 study, as well as other noteworthy studies from the conference, on VJ Oncology’s GU Cancer Sessions podcast. Listen here.
The Weill Cornell Medicine GU Oncology Program is dedicated to advancing genitourinary cancer research, improving clinical outcomes, and enhancing the quality of life for all those affected by these diseases. Our team of physicians and scientists continue to conduct research throughout the year aimed at enhancing the treatment possibilities and understanding of how we can best manage and tackle GU cancers.